Is Graphene Elvis or the Russell Brand of Materials?
Image credit http://www.entertainmentwise.com
If there were a Rolling Stone magazine equivalent for the materials science set, graphene would be on the cover. Graphene consists of “single-atom–thick sheets of carbon prized for its off-the-charts ability to conduct electrons and for being all but transparent” (Service). Graphene, like Russell Brand, has some intriguing qualities: it’s extremely strong and highly conductive, which along with its transparency, make it an attractive alternative for use as a transparent conductor. Everything from computer displays and flat panel TVs to ATM touch screens and solar cells use transparent conductors these days, and finding a material that is strong, thin, and flaw-free has been a challenge.
Image credit http://www.lbl.gov
According to Moore’s Law, the density of transistors on an integrated circuit doubles every two years. Silicon and “other existing transistor materials are thought to be close to the minimum size where they can remain effective. Graphene transistors can potentially run at faster speeds and cope with higher temperatures. Graphene could be the solution to ensuring computing technology to continue to grow in power whilst shrinking in size, extending the life of Moore’s law by many years” (Science Daily). In other words, graphene might be about to drop Jailhouse Rock.
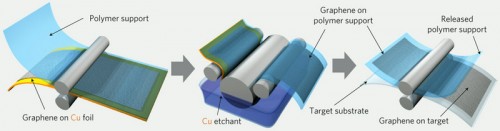
Since scientists first isolated graphene in 2004, they’ve struggled to produce the carbon sheets in sizes large enough to be useful. Last year, a group led by University of Texas, Austin chemist Rodney Ruoff grew graphene squares one centimeter square atop flexible copper foils. A few days ago, a group of researchers led by Jong-Hyun Ahn and Byung Hee Hong of Sungkyunkwan University in South Korea submitted a report in Nature Nanotechnologydescribing their efforts to scale up the approach taken by the Texas team to make graphene sheets large enough for full-screen displays (Service).
The graphene microchip. (Credit: Photo / Donna Coveney)
Ahn and Hong et al used chemical vapor depositionto grow graphene on large sheets of copper foil. A thin adhesive polymer was layered on top of the graphene, and then the copper backing was dissolved away. “Peeling off the adhesive polymer gave them a single graphene sheet. To make their film stronger, they repeated the initial steps, layering four sheets of graphene atop one another. The researchers then chemically treated their graphene sandwich with nitric acid to improve its electrical conductivity. The film allowed 90% of light to pass through and had an electrical resistance lower than that of the standard transparent conductor made from indium tin oxide (ITO)” (Service).
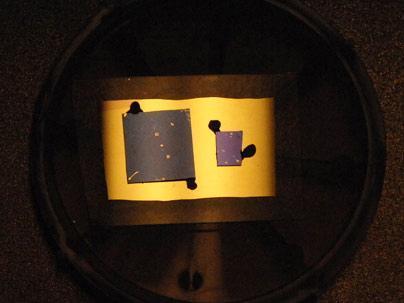
Credit: Jong-Hyun Ahn et al., Nature Nanotechnology, Advance Online Publication (2010).
Graphene could be used to make more efficient/cheaper solar cells, better large screen displays for electronics, and so on. If the larger sheet sizes pan out, we might just be looking at the Elvis of materials. Time will tell.
WU XING:
I’m filing graphene under EARTH because it’s carbon-based.
Cited:
Science Daily. “Breakthrough in Developing Super-Material Graphene.” 01/19/10. Accessed 06/23/10. URL.
Service, Robert F. “Graphene Finally Goes Big.” Science Now. 06/20/10. Accessed 06/23/10. URL.
















[…] post: Is Graphene Elvis or the Russell Brand of Materials? | ARCHITERIALS By admin | category: Sungkyunkwan University | tags: approach, efforts, few-days, nature, […]
No one will beat the king which is Elvis Presley.”–
[…] and semi-conductor properties that put silicon to shame and that even rival super-material graphene. The material had been used in the past as an additive in lubricants and as a component of steel […]
Leave a Wordpress Comment:
Ads
Watch ARCHITERIALS Videos on vimeo
Like on Facebook
Twitter
Flickr
Hit Counter
Ads
Blogs
Green
Journals/Publications
Materials
Network/News
Offices/People
Resources
Science
Pages
Archive
RSS and Email Subscriptions
Tag Cloud
3D 3D printer AB FAB academic acid acrylic actuated matter adaptive adhesive adsorption aerogel air air conditioning alloy aluminum amnh antibacterial antifungal ants april fool's architecture architecture robot artificial skin autonomous aviation awesome bacteria bamboo bananas beer bench bend bending biennale biocomputing biodegradable biodegradeable biomaterials biomimetics biomimicry biominerals biopolymer birds blast blast-resistant block blocks blogs Bloom Box brazil brick bubbles bucky bulk metallic glass butterfly calera canvas carbon carbon fiber carbon nanotubes carpet cars ceiling cellulose cement ceramic chain link chair charcoal charlie sheen chemicals chiller clay cloth cloud cmu coils color color-changing communication compound computer concrete condensation conducting conductive context cool coral cracks crystal cyborg demakersvan design digifab dirt disaster dna dror drywall dutch dynamic EAP earth ecocradle ecolect e coli ecology ecoresin ecovative elastic electric electricity electrochromic electroluminescent electronic energy energy recovery environment evaporative cooling experiment fabric fabrication facade fiber fiberglass fiber optic fiber optics fibers film FIRE flexible flickr fly ash foam fungus furniture garbage gel geodesic dome geometry gfrp gilgamesh glass glass fiber glow glue gold graphene green greensulate gsapp gypsum hard heat heavy heidi klum helix hemp hexagon hidden high performance hive honeybee humidity ice India ink insulation interference Internet inventables invisible invisible ink jello jellyfish just add water kevlar kinetic korea lace lamboo laser lattice leaves LED leed LEGO light light emitting light transmitting liquid lo mein london Loop.pH machines magic magnetic marine material materials meatball melting memory metabolic engineering METAL metal panel metamaterial micro microsensor microtools military milk MIT moisture multi-layer mushrooms mycelium nano nanogel nanotech nanotubes NASA new noise non-metallic oil OLED OMA ostrich oysters packaging paint panel panels paper paperfoam paraffin wax particles particulates petroleum phase change phosphorescent pink PLA plastic platinum pm-10 poetry pollution polymer polymers porcelain power precast printed printing protein public quadror radiant rain rammed earth reclaimed recycled reflective refracting Rem Koolhaas resin robot robotics roof rubber rugged sand sealant sealer search segmented self-healing sensor shape memory silica silicon silk skin skittles slats smog soft solar solar cell solar cells solar paint solid solid state lighting sound spider spider glue spray stabilized sand stone stretch stretchable strong structural structure studio dror sun sunglasses super supercritical sustainable switzerland tags tape technology TED tensile TEXAS textile textiles texture thermal thermochromatic thin thin film thread tiger stone tile tiles timber tio2 tires toaster tokujin yoshioka touch touch-sensitive toxic transparent t shirts tulip ultra-thin university of akron university of connecticut upcycling virus voc wall wallpaper WATER web wet whale wires WOOD woodwool wool workshop
WP Cumulus Flash tag cloud by Roy Tanck and Luke Morton requires Flash Player 9 or better.
Recent Comments
Ads
Recent Posts
Most Commented
Most Viewed